Electromagnetic Spectrum
The entire range of electromagnetic radiation, which includes, in order of increasing frequency and decreasing wavelength, radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, x-rays, and gamma rays. (General Physics) the complete range of electromagnetic radiation from.
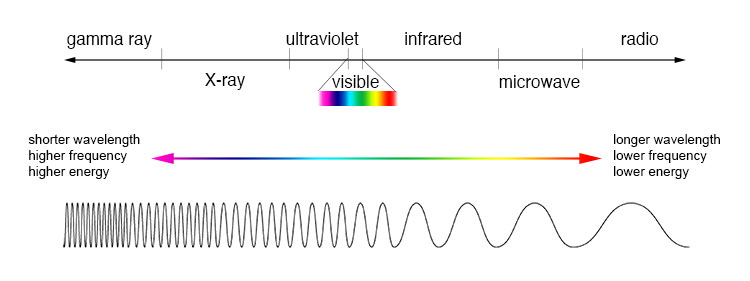
A diagram of the electromagnetic spectrum, showing various properties across the range of frequencies and wavelengths The types of electromagnetic radiation are broadly classified into the following classes (regions, bands or types):. Gamma radiation. X-ray radiation. Ultraviolet radiation. Visible radiation. Infrared radiation. Terahertz radiation.
Microwave radiation. Radio waves This classification goes in the increasing order of wavelength, which is characteristic of the type of radiation. While, in general, the classification scheme is accurate, in reality there is often some overlap between neighboring types of electromagnetic energy. For example, SLF radio waves at 60 Hz may be received and studied by astronomers, or may be ducted along wires as electric power, although the latter is, in the strict sense, not electromagnetic radiation at all (see ). Note that there are no precisely defined boundaries between the bands of the electromagnetic spectrum; rather they fade into each other like the bands in a rainbow (which is the sub-spectrum of visible light). Radiation of each frequency and wavelength (or in each band) has a mix of properties of the two regions of the spectrum that bound it.
For example, red light resembles infrared radiation in that it can excite and add energy to some and indeed must do so to power the chemical mechanisms responsible for and the working of the. The distinction between X-rays and gamma rays is partly based on sources: the photons generated from or other nuclear and subnuclear/particle process, are always termed gamma rays, whereas X-rays are generated by transitions involving highly energetic inner atomic electrons. In general, nuclear transitions are much more energetic than electronic transitions, so gamma-rays are more energetic than X-rays, but exceptions exist. By analogy to electronic transitions, transitions are also said to produce X-rays, even though their energy may exceed 6 megaelectronvolts (0.96 pJ), whereas there are many (77 known to be less than 10 keV (1.6 fJ)) low-energy nuclear transitions (e.g., the 7.6 eV (1.22 aJ) nuclear transition of -229), and, despite being one million-fold less energetic than some muonic X-rays, the emitted photons are still called gamma rays due to their nuclear origin. The convention that EM radiation that is known to come from the nucleus, is always called 'gamma ray' radiation is the only convention that is universally respected, however. Many astronomical sources (such as ) are known to be too energetic (in both intensity and wavelength) to be of nuclear origin. Quite often, in high energy physics and in medical radiotherapy, very high energy EMR (in the 10 MeV region)—which is of higher energy than any nuclear gamma ray—is not called X-ray or gamma-ray, but instead by the generic term of 'high energy photons.'
The region of the spectrum where a particular observed electromagnetic radiation falls, is -dependent (due to the for light), so EM radiation that one observer would say is in one region of the spectrum could appear to an observer moving at a substantial fraction of the speed of light with respect to the first to be in another part of the spectrum. For example, consider the. It was produced, when matter and radiation decoupled, by the de-excitation of hydrogen atoms to the ground state. These photons were from transitions, putting them in the ultraviolet (UV) part of the electromagnetic spectrum. Now this radiation has undergone enough cosmological to put it into the microwave region of the spectrum for observers moving slowly (compared to the speed of light) with respect to the cosmos. Rationale for names Electromagnetic radiation interacts with matter in different ways across the spectrum. These types of interaction are so different that historically different names have been applied to different parts of the spectrum, as though these were different types of radiation.
Thus, although these 'different kinds' of electromagnetic radiation form a quantitatively continuous spectrum of frequencies and wavelengths, the spectrum remains divided for practical reasons related to these qualitative interaction differences. Electromagnetic radiation interaction with matter Region of the spectrum Main interactions with matter Collective oscillation of charge carriers in bulk material. An example would be the oscillatory travels of the electrons in an. Through far Plasma oscillation, molecular rotation Near Molecular vibration, plasma oscillation (in metals only) Molecular electron excitation (including pigment molecules found in the human retina), plasma oscillations (in metals only) Excitation of molecular and atomic valence electrons, including ejection of the electrons Excitation and ejection of core atomic electrons, (for low atomic numbers) Energetic ejection of core electrons in heavy elements, (for all atomic numbers), excitation of atomic nuclei, including dissociation of nuclei High-energy Creation of. At very high energies a single photon can create a shower of high-energy particles and antiparticles upon interaction with matter. Types of radiation Radio frequency. Main articles:, and waves are emitted and received by, which consist of conductors such as metal rod.
In artificial generation of radio waves, an electronic device called a generates an which is applied to an antenna. The oscillating electrons in the antenna generate oscillating and that radiate away from the antenna as radio waves. In reception of radio waves, the oscillating electric and magnetic fields of a radio wave couple to the electrons in an antenna, pushing them back and forth, creating oscillating currents which are applied to a. Earth's atmosphere is mainly transparent to radio waves, except for layers of charged particles in the which can reflect certain frequencies.
Radio waves are extremely widely used to transmit information across distances in systems such as,. In a radio communication system, a radio frequency current is with an information-bearing in a transmitter by varying either the amplitude, frequency or phase, and applied to an antenna. The radio waves carry the information across space to a receiver, where they are received by an antenna and the information extracted by in the receiver. Radio waves are also used for navigation in systems like (GPS) and, and locating distant objects in. They are also used for, and for industrial heating.
The use of the is strictly regulated by governments, coordinated by a body called the (ITU) which to different users for different uses. Plot of Earth's atmospheric transmittance (or opacity) to various wavelengths of electromagnetic radiation. Are radio waves of short, from about 10 centimeters to one millimeter, in the and frequency bands. Microwave energy is produced with and tubes, and with devices such as. Although they are emitted and absorbed by short antennas, they are also absorbed by, coupling to vibrational and rotational modes, resulting in bulk heating.
Unlike higher frequency waves such as and which are absorbed mainly at surfaces, microwaves can penetrate into materials and deposit their energy below the surface. This effect is used to heat food in, and for industrial heating and medical. Microwaves are the main wavelengths used in, and are used for, and technologies such as, although this is at intensity levels unable to cause thermal heating. The copper cables which are used to carry lower frequency radio waves to antennas have excessive power losses at microwave frequencies, and metal pipes called are used to carry them. Although at the low end of the band the atmosphere is mainly transparent, at the upper end of the band absorption of microwaves by atmospheric gasses limits practical propagation distances to a few kilometers. Terahertz radiation. Main article: Terahertz radiation is a region of the spectrum between far infrared and microwaves.
Until recently, the range was rarely studied and few sources existed for microwave energy at the high end of the band (sub-millimeter waves or so-called ), but applications such as imaging and communications are now appearing. Scientists are also looking to apply terahertz technology in the armed forces, where high-frequency waves might be directed at enemy troops to incapacitate their electronic equipment. Terahertz radiation is strongly absorbed by atmospheric gases, making this frequency range useless for long distance communication.
Infrared radiation. Main article: The part of the electromagnetic spectrum covers the range from roughly 300 GHz to 400 THz (1 mm - 750 nm).
It can be divided into three parts:. Far-infrared, from 300 GHz to 30 THz (1 mm – 10 μm). The lower part of this range may also be called microwaves or terahertz waves. This radiation is typically absorbed by so-called rotational modes in gas-phase molecules, by molecular motions in liquids, and by in solids.
The water in Earth's atmosphere absorbs so strongly in this range that it renders the atmosphere in effect opaque. However, there are certain wavelength ranges ('windows') within the opaque range that allow partial transmission, and can be used for astronomy. The wavelength range from approximately 200 μm up to a few mm is often referred to as, reserving far infrared for wavelengths below 200 μm. Mid-infrared, from 30 to 120 THz (10–2.5 μm). Hot objects ( radiators) can radiate strongly in this range, and human skin at normal body temperature radiates strongly at the lower end of this region.
This radiation is absorbed by molecular vibrations, where the different atoms in a molecule vibrate around their equilibrium positions. This range is sometimes called the fingerprint region, since the mid-infrared absorption spectrum of a compound is very specific for that compound.
Near-infrared, from 120 to 400 THz (2,500–750 nm). Physical processes that are relevant for this range are similar to those for visible light. The highest frequencies in this region can be detected directly by some types of photographic film, and by many types of solid state for and videography. Visible radiation (light).
Main article: Above infrared in frequency comes. The emits its peak power in the visible region, although integrating the entire emission power spectrum through all wavelengths shows that the Sun emits slightly more infrared than visible light. By definition, visible light is the part of the EM spectrum the is the most sensitive to. Visible light (and near-infrared light) is typically absorbed and emitted by electrons in molecules and atoms that move from one energy level to another. This action allows the chemical mechanisms that underlie human vision and plant photosynthesis. The light that excites the human is a very small portion of the electromagnetic spectrum. A shows the optical (visible) part of the electromagnetic spectrum; infrared (if it could be seen) would be located just beyond the red side of the rainbow with appearing just beyond the violet end.
Electromagnetic radiation with a between 380 and 760 nm (400–790 terahertz) is detected by the human eye and perceived as visible light. Other wavelengths, especially near infrared (longer than 760 nm) and ultraviolet (shorter than 380 nm) are also sometimes referred to as light, especially when the visibility to humans is not relevant. White light is a combination of lights of different wavelengths in the visible spectrum. Passing white light through a prism splits it up into the several colors of light observed in the visible spectrum between 400 nm and 780 nm. If radiation having a frequency in the visible region of the EM spectrum reflects off an object, say, a bowl of fruit, and then strikes the eyes, this results in of the scene. The brain's visual system processes the multitude of reflected frequencies into different shades and hues, and through this insufficiently-understood psychophysical phenomenon, most people perceive a bowl of fruit.
At most wavelengths, however, the information carried by electromagnetic radiation is not directly detected by human senses. Natural sources produce EM radiation across the spectrum, and technology can also manipulate a broad range of wavelengths. Transmits light that, although not necessarily in the visible part of the spectrum (it is usually infrared), can carry information.
The modulation is similar to that used with radio waves. Ultraviolet radiation.
The amount of penetration of UV relative to altitude in Earth's Next in frequency comes (UV). The wavelength of UV rays is shorter than the violet end of the but longer than the X-ray. UV is the longest wavelength radiation whose photons are energetic enough to atoms, separating from them, and thus causing. Short wavelength UV and the shorter wavelength radiation above it (X-rays and gamma rays) are called, and exposure to them can damage living tissue, making them a health hazard.
UV can also cause many substances to glow with visible light; this is called. At the middle range of UV, UV rays cannot ionize but can break chemical bonds, making molecules unusually reactive., for example, is caused by the disruptive effects of middle range UV radiation on, which is the main cause of. UV rays in the middle range can irreparably damage the complex molecules in the cells producing making it a very potent. The Sun emits significant UV radiation (about 10% of its total power), including extremely short wavelength UV that could potentially destroy most life on land (ocean water would provide some protection for life there). However, most of the Sun's damaging UV wavelengths are absorbed by the atmosphere before they reach the surface.
The higher energy (shortest wavelength) ranges of UV (called 'vacuum UV') are absorbed by nitrogen and, at longer wavelengths, by simple diatomic in the air. Most of the UV in the mid-range of energy is blocked by the ozone layer, which absorbs strongly in the important 200–315 nm range, the lower energy part of which is too long for ordinary in air to absorb. This leaves less than 3% of sunlight at sea level in UV, with all of this remainder at the lower energies. The remainder is UV-A, along with some UV-B. The very lowest energy range of UV between 315 nm and visible light (called UV-A) is not blocked well by the atmosphere, but does not cause sunburn and does less biological damage. However, it is not harmless and does create oxygen radicals, mutations and skin damage.
See for more information. Main article: After UV come, which, like the upper ranges of UV are also ionizing. However, due to their higher energies, X-rays can also interact with matter by means of the. Hard X-rays have shorter wavelengths than soft X-rays and as they can pass through many substances with little absorption, they can be used to 'see through' objects with 'thicknesses' less than that equivalent to a few meters of water. One notable use is diagnostic X-ray imaging in medicine (a process known as ). X-rays are useful as probes in high-energy physics. In astronomy, the accretion disks around and emit X-rays, enabling studies of these phenomena.
X-rays are also emitted by the of stars and are strongly emitted by some types of. However, must be placed outside the Earth's atmosphere to see astronomical X-rays, since the great depth of the is opaque to X-rays (with areal density of 1000 grams per cm 2), equivalent to 10 meters thickness of water. This is an amount sufficient to block almost all astronomical X-rays (and also astronomical gamma rays—see below). Main article: After hard X-rays come, which were discovered by in 1900. These are the most energetic, having no defined lower limit to their wavelength. In they are valuable for studying high-energy objects or regions, however as with X-rays this can only be done with telescopes outside the Earth's atmosphere. Gamma rays are used experimentally by physicists for their penetrating ability and are produced by a number of.
They are used for of foods and seeds for sterilization, and in medicine they are occasionally used in. More commonly, gamma rays are used for diagnostic imaging in, an example being. Gsa h10a drivers for mac. The wavelength of gamma rays can be measured with high accuracy through the effects of. ^ December 5, 2013, at the. – lecture slides. ^ Elert, Glenn. Retrieved 2010-10-16.
Retrieved 2010-10-16. A.; Godse, A. Technical Publications. ^ Mehta, Akul. Retrieved 2011-11-08. Haitel, Gary (2014-05-15).
Cool Cosmos Classroom activities. Archived from on 2012-02-25. Retrieved 4 March 2013.
He directed sunlight through a glass prism to create a spectrum and then measured the temperature of each colour. He found that the temperatures of the colors increased from the violet to the red part of the spectrum. Herschel decided to measure the temperature just beyond the red of the spectrum in a region where no sunlight was visible. To his surprise, he found that this region had the highest temperature of all. Davidson, Michael W.
The Florida State University. Retrieved 5 March 2013.
Ritter hypothesized that there must also be invisible radiation beyond the violet end of the spectrum and commenced experiments to confirm his speculation. He began working with silver chloride, a substance decomposed by light, measuring the speed at which different colors of light broke it down. Ritter demonstrated that the fastest rate of decomposition occurred with radiation that could not be seen, but that existed in a region beyond the violet.
Ritter initially referred to the new type of radiation as chemical rays, but the title of ultraviolet radiation eventually became the preferred term. Mohr, Peter J.; Taylor, Barry N.; Newell, David B. 80 (2): 633–730.:. J.; Ransom, S. Retrieved 2008-01-05.
A.; Allen, B.; Berley, D.; Blaufuss, E.; Casanova, S.; Chen, C.; Coyne, D. G.; Delay, R. S.; Dingus, B. L.; Ellsworth, R. W.; Fleysher, L.; Fleysher, R.; Gebauer, I.; Gonzalez, M. M.; Goodman, J.
A.; Hays, E.; Hoffman, C. M.; Kolterman, B. E.; Kelley, L. A.; Lansdell, C.
P.; Linnemann, J. T.; McEnery, J. E.; Mincer, A. I.; Moskalenko, I. V.; Nemethy, P.; Noyes, D.; Ryan, J.
Electromagnetic Spectrum Frequency
M.; Samuelson, F. W.; Saz Parkinson, P. 'Discovery of TeV Gamma-Ray Emission from the Cygnus Region of the Galaxy'.
The Astrophysical Journal. 658 (1): L33–L36.:. Feynman, Richard; Leighton, Robert; Sands, Matthew (1963). The Feynman Lectures on Physics, Vol.1. USA: Addison-Wesley.
L'Annunziata, Michael; Baradei, Mohammad (2003). Academic Press. Grupen, Claus; Cowan, G.; Eidelman, S. D.; Stroh, T. Astroparticle Physics. slac-pub-0335 (1967).
Retrieved 2010-10-16. March 6, 2005. Archived from on 6 January 2010. Retrieved 2010-09-27. Retrieved 2009-11-12. Koontz, Steve (26 June 2012).
NASA/MIT Workshop. See pages I-7 (atmosphere) and I-23 (for water). External links Wikimedia Commons has media related to. Radio spectrum allocations resource). (from Australian Communications and Media Authority). (from ).
— Covering the range 3 kHz to 300 GHz (from ). (from, which inherited the 's duties, pdf format).

– Very complete and customizable. – Only approximately right.
Electromagnetic Spectrum Energy
(992 kB).
Comments are closed.